Leave No Clot Behind: The Role of Retained Clot in Post Operative Effusions After Cardiac Surgery
Drainage systems are used to evacuate blood from around the heart and lungs after cardiac surgery. When the drainage capacity is impaired, for example by obstruction from chest tube clots, then blood is retained around the heart (pericardial space) and lungs (pleural spaces). Blood that is retained in these spaces causes a host of complications called Retained Blood Syndrome (RBS), often impairing patient recovery.
Retained blood clot drives the production of effusions
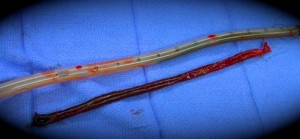 Blood that pools turns to clot. What is the fate of these clots in the pleural and pericardial spaces? It depends on the volume and location. Large volume clots must be surgically evacuated in a re-exploration procedure in the early days after surgery. Smaller, more focal clots that compress the heart can lead to hemodynamic compromise (pericardial tamponade) that can threaten the patient’s life. These can require a subsequent drainage procedure as well.
Blood that pools turns to clot. What is the fate of these clots in the pleural and pericardial spaces? It depends on the volume and location. Large volume clots must be surgically evacuated in a re-exploration procedure in the early days after surgery. Smaller, more focal clots that compress the heart can lead to hemodynamic compromise (pericardial tamponade) that can threaten the patient’s life. These can require a subsequent drainage procedure as well.
What about smaller clot collections that are not clinically recognized in the early days after surgery? A number of studies chronicle the reabsorption process of blood in the pleural and pericardial spaces after surgery and suggest that there is an initial breakdown period where the clot contracts and the more solid elements are separated from the fluid elements. This results in a bloody fluid production that is recognizable clinically as pericardial or pleural effusions, and is seen in between 60% and 80% of patients after heart surgery.1, 2 Between 10% and 15% of these patients required a pleural drainage procedure during recovery after cardiac surgery.1, 2, 3 When this fluid is examined under the microscope, there are bloody elements as well as inflammatory elements, a finding that correlates with experimental studies that show the pleural or pericardial surfaces remain inflamed throughout the acute, subacute, and chronic phases.3 This is no surprise as the thrombin contained in the clot is highly pro-inflammatory.4 This sets up a situation where fluid production, driven in part by vascular endothelial growth factor (VEGF), can continue as the inflammation flares initially, and then simmers before it goes away.5,6
Effusions are common and limit the speed and completeness of recovery
There is a growing appreciation that this may impact the high number of patients who have slow recoveries and even require subsequent fluid drainage procedures and/or readmission after heart surgery. Currently, nearly 1 in 5 cardiac surgery patients are readmitted within 30 days of discharge, and approximately 20% of readmissions are caused by pleural or pericardial effusions.
Preventing Retained Blood Can Reduce Effusions
Ultimately, becoming more aggressive about “leaving no clot behind” appears to be a strategy that may reduce these complications that impact hospital outcomes and increase long term costs of recovery. This is best accomplished by preventing chest tube clogging so that shed mediastinal blood can be maximally evacuated while the patient is still bleeding. Numerous studies have shown using PleuraFlowACT prevents chest tube clogging. When this is done in the ICU when the patient is still bleeding, active clearance can reduce reexploration for bleeding, effusions, POAF and other complications.11-23 For example, Sirch and colleagues demonstrated that utilizing PleuraFlow ACT in a defined preventive protocol has been shown to reduce RBS by 43% and POAF by 33%.22This was driven by a large drop in the number of pleural effusosn. This also included a significant reduction in the time on the ventilator postoperatively in the ICU. There have been several studies showing that active clearance of chest tubes reduces the need for take back for re exploration by up 55% to 100%.19,20 This includes a randomized study from Montreal showing a 75% reduction and one Washington University showing a 100% reduction. Simlarly these patients had fewer effusions that needed to be drained. Likewise Baribeau and colleagues recently showed not only fewer postoperative bloody pleural effusions, but also less AKI, fewer post op infections and a marked reduction in POAF. In this clinical trial they also noted a reduced time in the ICU and reduced hospital costs with active clearance of chest tubes using PleuraFlow.23
Expert Consensus Supports Active Clearance of Chest Tubes to Minimize Retained Blood
Given the growing evidence for active chest tube maintenance of chest tube patency after cardiac surgery, and the mounting expert consensus against chest tube stripping, more and more programs are making the switch to implement PleuraFlow ACT as part of the routine post op care in the ICU with the goal of reducing complications and reducing costs of care. In the most recent ERAS Cardiac Society active clearance of chest tubes was given a Level 1 recommendation based on published evidence that it can help reduce retained blood.24
For more information about how developing chest tube patency protocols and PleuraFlow ACT can help your facility address this common and costly problem, contact us.
- Vargas FS, Cukier A, Hueb W, Teixeira LR, Light RW. Relationship between pleural effusion and pericardial involvement after myocardial revascularization. Chest. 1994;105(6):1748-52.
- Light RW, Rogers JT, Moyers JP, Lee YC, Rodriguez RM, Alford WC, Jr., et al. Prevalence and clinical course of pleural effusions at 30 days after coronary artery and cardiac surgery.American Journal of Respiratory and Critical Care Medicine. 2002;166(12 Pt 1):1567-71.
- Sadikot RT, Rogers JT, Cheng DS, Moyers P, Rodriguez M, Light RW. Pleural fluid characteristics of patients with symptomatic pleural effusion after coronary artery bypass graft surgery. Archives of Internal Medicine.2000;160(17):2665-8.
- Hott JW, Sparks JA, Godbey SW, Antony VB. Mesothelial cell response to pleural injury: thrombin-induced proliferation and chemotaxis of rat pleural mesothelial cells. American Journal of Respiratory Cell and Molecular Biology. 1992;6(4):421-5.
- Marchi E, Broaddus VC. Mechanisms of pleural liquid formation in pleural inflammation. Current opinion in pulmonary medicine. 1997;3(4):305-9.
- Grove CS, Lee YC. Vascular endothelial growth factor: the key mediator in pleural effusion formation. Current Opinion in Pulmonary Medicine. 2002;8(4):294-301.
- Karimov JH, Gillinov AM, Schenck L, et al. Incidence of chest tube clogging after cardiac surgery: a single-center prospective observational study. Eur J Cardiothorac Surg. 2013;44(6):1029-1036. doi:1093/ejcts/ezt140
- Shalli S, Saeed D, Fukamachi K, et al. Chest tube selection in cardiac and thoracic surgery: a survey of chest tube-related complications and their management. J Card Surg. 2009;24(5):503-509. doi:1111/j.1540-8191.2009.00905.x
- Balzer F, von Heymann C, Boyle EM, Wernecke KD, Grubitzsch H, Sander M. Impact of retained blood requiring reintervention on outcomes after cardiac surgery. J Thorac Cardiovasc Surg. 2016;152(2):595-601.e4. doi:1016/j.jtcvs.2016.03.086
- Tauriainen TKE, Morosin MA, Airaksinen J, Biancari F. Outcome after procedures for retained blood syndrome in coronary surgery. Eur J Cardiothorac Surg. 2017;51(6):1078-1085. doi:1093/ejcts/ezx015
- St-Onge S, Perrault LP, Demers P, et al. Pericardial blood as a trigger for postoperative atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2018;105(1):321-328. doi:1016/j.athoracsur.2017.07.045
- Boyle EM, Jr, Gillinov AM, Cohn WE, et al. Retained blood syndrome after cardiac surgery: a new look at an old problem. Innovations (Phila) 2015;10(5):296–303. doi: 10.1097/IMI.0000000000000200.
- Day TG, Perring RR, Gofton K. Is manipulation of mediastinal chest drains useful or harmful after cardiac surgery? Interact Cardiovasc Thorac Surg. 2008;7(5):888-890. doi:1510/icvts.2008.185413
- Halm MA. To strip or not to strip? physiological effects of chest tube manipulation. Am J Crit Care. 2007;16(6):609-612.
- Boyacıoğlu K, Kalender M, Özkaynak B, Mert B, Kayalar N, Erentuğ V. A new use of Fogarty catheter: chest tube clearance. Heart Lung Circ. 2014;23(10):e229-e230. doi:1016/j.hlc.2014.04.255
- Shiose A, Takaseya T, Fumoto H, Arakawa Y, Horai T, Boyle EM, Gillinov AM, Fukamachi K. Improved drainage with active chest tube clearance. Interact Cardiovasc Thorac Surg. 2010;10(5):685–8. DOI: 1510/icvts.2009.229393
- Arakawa Y, Shiose A, Takaseya T, Fumoto H, Kim HI, Boyle EM, Gillinov AM, Fukamachi K. Superior chest drainage with an active tube clearance system: evaluation of a downsized chest tube. Ann Thorac Surg. 2011;91(2):580–3. DOI: 1016/j.athoracsur.2010.10.018.
- Perrault LP, Pellerin M, Carrier M, et al. The PleuraFlow Active chest tube clearance system: initial clinical experience in adult cardiac surgery. Innovations (Phila). 2012;7(5):354-358. org/10.1097/imi.0b013e31827e2b4d
- Grieshaber P, Heim N, Herzberg M, Niemann B, Roth P, Boening A. Active chest tube clearance after cardiac surgery is associated with reduced reexploration rates. Ann Thorac Surg. 2018;105(6):1771-1777. doi:1016/j.athoracsur.2018.01.002
- Maltais S, Davis ME, Haglund NA, et al. Active clearance of chest tubes reduces re-exploration for bleeding after ventricular assist device implantation. ASAIO J. 2016;62(6):704-709. doi:1097/MAT.0000000000000437
- St-Onge S, Ben Ali W, Bouhout I, et al. Examining the impact of active clearance of chest drainage catheters on postoperative atrial fibrillation. J Thorac Cardiovasc Surg. 2017;154(2):501-508. doi:1016/j.jtcvs.2017.03.046
- Sirch J, Ledwon M, Püski T, Boyle EM, Pfeiffer S, Fischlein T. Active clearance of chest drainage catheters reduces retained blood. J Thorac Cardiovasc Surg. 2016;151(3):832-838.e2. doi:1016/j.jtcvs.2015.10.015
- Baribeau Y, Westbrook B, Baribeau Y, et al. Active clearance of chest tubes is associated with reduced postoperative complications and costs after cardiac surgery: a propensity matched analysis. J Cardiothorac Surg. 2019;14(1):192. org/10.1186/s13019-019-0999-3
- Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for perioperative care in cardiac surgery: Enhanced recovery after surgery society recommendations. JAMA Surg.2019; 154: 755-766. doi:10.1001/jamasurg.2019.1153